Implementing E6 R3: Data Changes
Regs and Guidance
OVERVIEW
Fast, secure, and easy to use, Ready Room replaces your slide decks, trackers, folders, video conferencing systems, and chat rooms with a single system to fully manage inspection requests from start to finish.

Regulatory inspections can be nerve-wracking. Days and weeks of questions, interviews, and requests for documentation. Make a mistake and you risk a delay, or worse, a finding. Most life sciences companies try to manage inspections with heavy-handed processes involving spreadsheet trackers, shared drives, chat rooms, and a great deal of finger crossing.
Ready Room puts an end to all that. Using the latest technology, Ready Room presents a simple and intuitive, drag and drop workflow that's synchronized across every team member's desktop. With simple request handling, unlimited document uploads, and a rich suite of collaboration tools, your team can easily handle anything that comes at them, regardless of whether they are local or remote.
Inspection Readiness
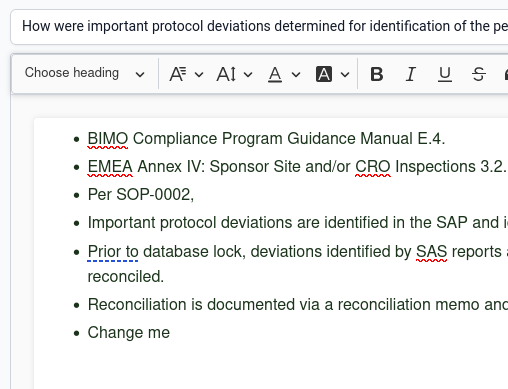
Throw away your slide decks and shared drives. Create and refine your storyboards in Ready Room starting with our "fill in the blank" GCP, GMP, and GVP templates. Manage storyboard development using a powerful multi-step, drag & drop workflow. Assign owners and collaborators to every stage of the workflow. Use rich text to create storyboards with images, tables, links, and more. Upload associated documentation and add metadata. When you're ready, SMEs can use our unique "flash card" feature to rehearse delivery. Share storyboards across inspections and click to turn them into inspection requests.
Inpection Management
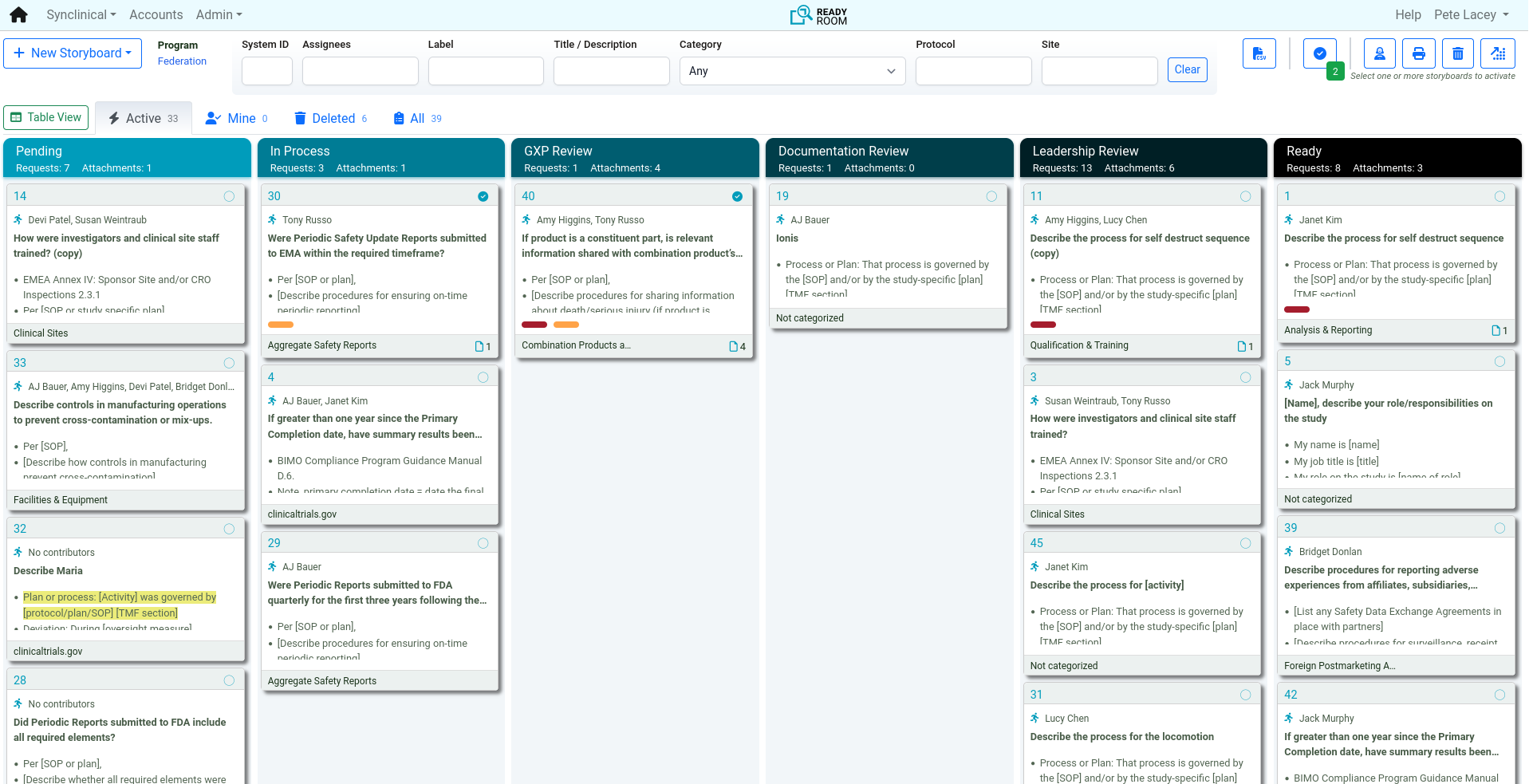
When the inspectors call, use Ready Room’s inspection management functionality to capture, assign, and manage requests through an intuitive drag & drop workflow. Enter formal responses and attach documents, QC deliverables before release, and deliver responses electronically to the inspector. The color-coded board lets the whole team visualize the status of each inspection request at a glance, while scribe notes, comments, video briefings, and chat facilitate communication.
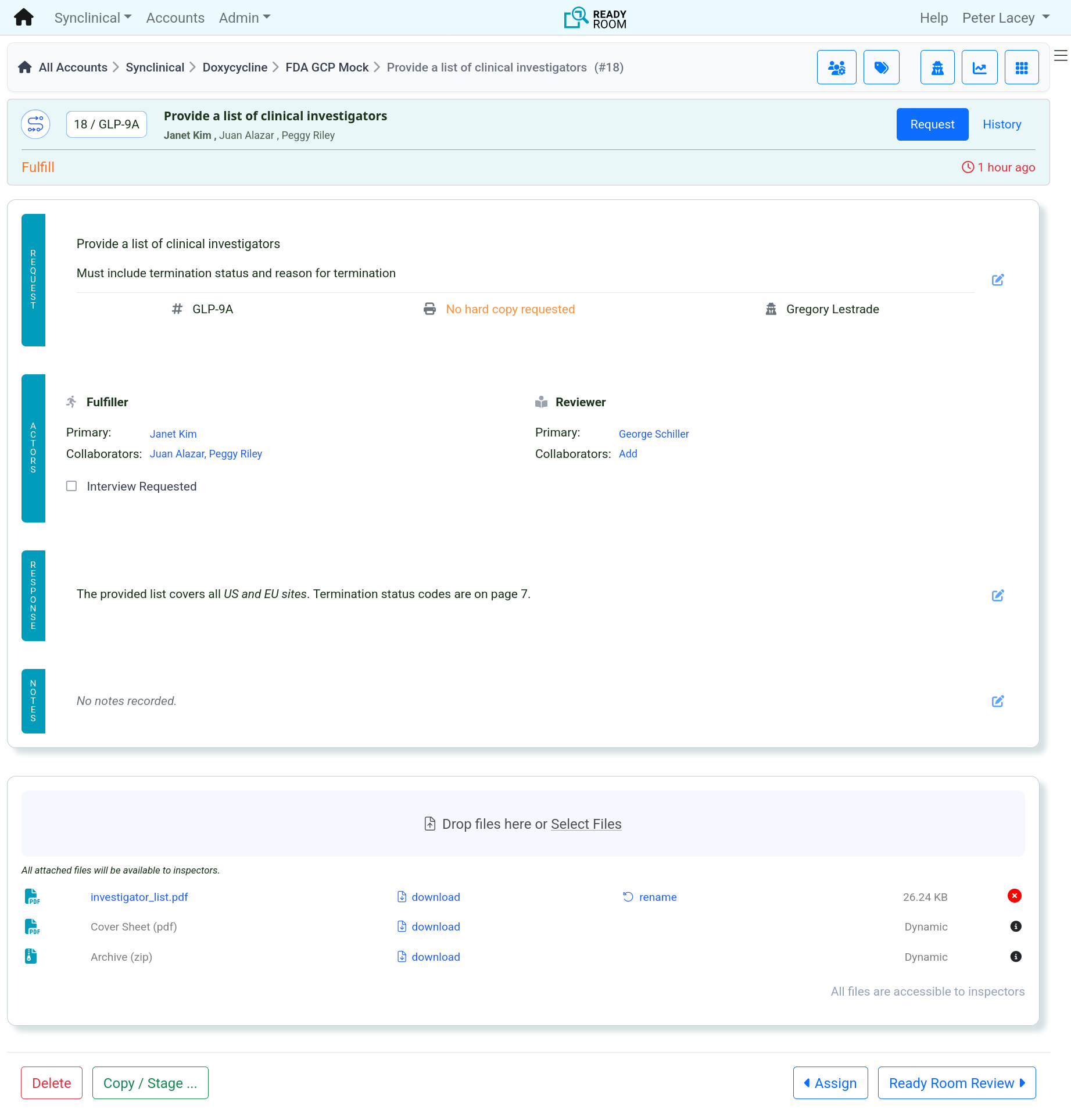
There's more to storyboards than PowerPoint
This brief video highlights Ready Room's inspection readiness capabilities.
Schedule a two week, full access, pilot for up to eight participants!
What our customers are saying
Biotech, pharmaceutical, medical device, CMOs, CROs, and laboratories big and small are getting ready with Ready Room.